- Company
- Products
- Technical report
- Indusrial cleaning
- Micro Joining and Assembly Technology
- Ultrasonic technology
- Trouble solution
- Cleaning
- Removing
- Attaching
- PCB Columns
Published on :
6.2 Deflux Chemicals
Soldering in electronics packaging uses flux in most cases. This use of flux evokes following demands:
a. cleaning of flux residue on PCBs after soldering,
b. cleaning of resin deposited in reflow furnace,
c. cleaning of flux branded on solder pallets, and
d. cleaning of flux attached on peripheral devices of fluxer, tools and jigs.
In this article we specifically define the chemicals for a. cleaning of flux residue on PCBs after soldering, which requires the most sophisticated cleaning technology of all, as 'deflux chemicals'. Meanwhile, we omit the general arguments and classifications on flux cleaning materials since a number of books have discussed about them already. 1)~3)
Current situation questions the significance of deflux, where joining technology plays an important role in electronics packaging, and increasingly stricter chemical substance regulations regarding the packaging scene calls for mitigation of energy and environmental burden. Moreover, ever higher density and speed of the circuits asks for even more sophisticated cleaning technology in order to lessen the disturbing impact of flux residue on circuit characteristics.
Therefore, this article explains the history, purpose and significance of deflux in the first half, and goes on to the latter half on existing technology and future issues concerning glycol ether based chemicals, the most popularly used deflux chemicals in the packaging scene.
Flux in early times comprising Hydrochloric acid demanded immediate water rinsing and drying because of its strong corrosiveness. Birth of rosin-based flux in 1960s improved the reliability after soldering, bringing us the concept of non-cleaning. In the late 1970s, however, when surface packaging became the mainstream with rapidly downsizing electronics, demands on deflux reincreased with higher aspiration for reliability on PCBs. This is when Du Pont introduced CFC-113, one of its industrialized fluorine compounds to the Japanese market as 'magical liquid' for it being nonflammable, fast-drying, insulative, chemically stable and safe for precision cleaning. This liquid is the so-called 'Freon', which became the standard for flux cleaning for about 20 years and established the status of flux cleaning in electronics packaging process until it was banned at the end of the year 1995 after being accused of causing the ozone depletion.
In the meantime, neither the necessity of deflux nor post-deflux circuit performance attracted much attention for the following reasons: Freon became reasonable as it spread in the market; the characteristic of Freon did not require the cleaning equipment furnish state-of-the-art technology but it be compact and inexpensive; and the whole Japanese market of electronics at the time was experiencing a rapid growth and expansion.
It was the phasing out of Freon and collapse of the Japanese bubble economy that triggered the rise of deflux business. Together with Freon, Trichloroethane, the chlorine solvent mainly used for metal cleaning, was banned to create a new market for cleaning of approximately 0.3 million tons per year. About 300 companies, mostly chemical manufacturers, entered this newly created market from 1988 to 1995.
Many veered to introduce the non-cleaning process during this warlike period, thanks to soldering manufacturers who have developed non-cleaning flux while the packaging industries once again giving serious consideration on the necessity and performance evaluation of deflux. Yet cleaning process is of the importance regarding in-vehicle equipment and high frequency parts that need to have high reliability, and high-density semiconductor packaging.
Companies that introduce deflux often aim to avoid the following residual flux-related problems:
When doing wire bonding or flip chip bonding in assembly and testing process, mere organic coating of flux residue on electrodes e.g. gold-plated pads is likely to cause bonding defect by weakening the bonding strength. Thus cleaning is important to remove the flux residue entirely. One thing to keep note is that wrong cleaning methods can result in chemicals residue compounds or flux reattachment, worsening the bonding defect.
Flux residue can create void when applying underfill or mold resin on the soldering surface in assembly and testing process; for example, its metal soaps and wax undermine wettability of resin or cause resin cure failure due to its amine compounds.
Besides flux residue, solder ball generated from slump and spatter in printing or preheating process should be removed to avoid electrical failure including leakage and short circuit. Solder ball is generated from rapid heating of solder when solvent gas in flux expands to spatter the flux and solder.
Since most kinds of current flux are able to provide high insulation reliability in terms of flux residue after soldering, rare cases demand cleaning to avoid insulation discredit. However, residue of water soluble flux, which is partially used, cannot offer insulation reliability unless immediately washed away with hot water and dried out because of its hygroscopicity and corrosiveness. Some water soluble flux requires cleaning with appropriate deflux chemicals since its post-soldering residue remains even after the hot water cleaning. Such cases are expected to increase with the trend of VOC elimination.
Flux residue, containing insulator of rosin, may attach to contacts such as connector to cause contact failure. Yet cleaning may not be effective or brings even worse results for U-shaped contacts of connector if the cleaning method is inappropriate.
Surface inspection to find blow holes, one of soldering defects, or to identify gloss of solder and fillet shape becomes difficult to carry out if the junction is covered with flux residue. Residual flux on fixture pins for in-circuit test or function test also diminishes the efficiency of electrical inspection by hindering connection.
Although deflux is introduced for the above-listed reasons, packaging sites have always discussed the option of non-cleaning method by reviewing packaging process and soldering conditions, or by improving the flux itself in order to achieve a better working environment and cost efficiency.
However, flux is a highly active material that plays the role to accelerate wettability with solder and alloy formation by chemically reacting with oxide, hydroxide, and carbonate on the surface of joined metal, turning them into fusible and soluble compounds e.g. metal oxide, metal complex, and organic acid metal soaps to add fluidity. Additionally, resins, including rosin to prevent exposed metal surface re-oxidation, react fiercely in soldering process and generate flux residue while being exposed to intense heat for organic materials of over 200°C (392°F). It is hard to imagine that stable electronic characteristic and circuit constant aimed by the circuit designer can be maintained with residual flux remained on PCBs. Moreover, the following reasons make it difficult for all the flux components to react completely: the level of joined metal surface oxidation varies on parts and materials; various shapes of resin parts with low thermal conductivity surround the junction; and heated condition differs when soldered. Soldering temperature rise due to recent inclination toward lead-free, too, has pressed the need to elevate flux activity in order to maintain its effectivity. Besides, halogen-free stream lead to more organic acids, being less active than halogen compounds which act vigorously with a little amount, added in the activator, making it almost impossible to maintain as good electrical characteristic as flux residue for PCB insulation materials.
Flux residue after soldering is a compound of various dielectric components with many contact surfaces, with flux being composed of resin including modified rosin which has relatively low polarity and high permittivity and of activator with high polarity and high permittivity(Fig6.2.1). In addition, with lower dielectric PCB materials adjusting to the recent trend of high frequency and high speed, residual flux as dielectric exists as stray capacitance between fine pitch circuits on the low permittivity boards to invoke interfacial polarization when put in an AC electric field.1)
Appropriate cleaning to remove flux residue is necessary to maintain primary circuit characteristics since electric characteristic of flux residue cannot follow that of PCBs as density, speed and frequency go higher onward.
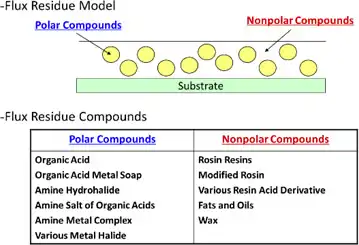
Deflux chemicals design is one of the most difficult in the field of designing industrial cleaners, since it has to fulfill a number of contradictory requirements and conditions listed as follows;
(1) Consistent PCB electrical characteristic before and after the cleaning process
(2) Extensive dissolvability of residual flux, the mixed compounds of largely varied polarities and solubility parameters (Fig 6.2.2)
(3) Little impact on PCB parts including electrical parts to be packaged
(4) Human/environmental safety in an appropriate working environment
(5) Inapplicability of concerned laws with strict use control
(6) Long life of cleaning with low agent deterioration
(7) Recyclability
(8) Sustainable supply with introducible costs
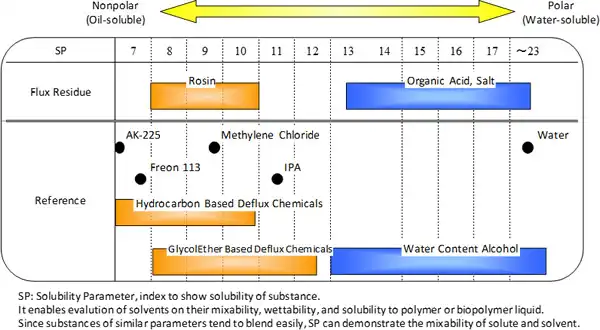
Among them, especially (2) is incompatible with (3) and (5) which makes it technically difficult to go together. Acceptable design is limited considering that the stricter chemical related laws constraint usable chemical materials and the influence on various parts on PCBs should be as little as possible. On the other hand, lead-free and halogen-free of soldering decreases residual flux dissolubility, demanding higher dissolvability of deflux chemicals. Generally high soluble materials are chemically active and likely to impact creatures, environment and electronic components.
Residual flux between fine pitches, under the electronic components for example, may swell due to high soluble deflux chemicals to unable cleaning, filling the space under the parts with a sticky liquid. Given these cases, adding a low soluble assistant should be considered in order to decrease the viscosity of the whole system. Deflux chemicals design must meet the contradictory requirements such as dissolvability of residual flux and low impacts on PCB electronic components, by using such technologies to mix several cleaning compounds. Combination with appropriate cleaning equipment is the key to match the ever more complicated needs of deflux for the packaging process, by maximizing the advantages of deflux chemicals and covering their disadvantages.
A variety of alternatives to Freon were considered after its disuse. Major deflux chemicals currently on the packaging market include Pine Alpha (Arakawa Chemical Industries, Ltd.), Cleanthrough (Kao Chemicals), and Microclean (Kaken Tech Co., Ltd.), all being glycol ether based chemicals.
Glycol ether, aforementioned in 6.1 Stencil Cleaners, consists of ethylene oxide (EO) or propylene oxide (PO) in addition to alcohol, butanol for instance which etherifies one or both of hydroxyl group of glycol, the dihydric alcohol. Table 6.2.1 shows some of glycol ethers produced for industrial use.
| Chemical Name | Abbr. | Boiling Point 1013hPa(C) |
Flash Point (C) |
|
|---|---|---|---|---|
| Ethylene Oxide | Diethylene glycol monobutyl ether | BDG | 230.6 | 120 |
| Triethylene glycol monobutyl ether | BTG | 271.2 | 156 | |
| Ethylene glycol monoisobutyl ether | iBG | 160.5 | 56.5 | |
| Ethylene glycol monohexyl ether | HeG | 208.0 | 102 | |
| Diethylene glycol monohexyl ether | HeDG | 259.1 | 141 | |
| Propylene Oxide | Propylene glycol monomethyl ether | PGM | 121.0 | 32 |
| Diethylene glycol monomethyl ether | MFDG | 187.2 | 76.5 | |
| Propylene glycol monopropyl ether | PFG | 149.8 | 48.5 | |
| Dipropylene glycol monopropyl ether | PFDG | 212.0 | 108 | |
| Propylene glycol monobutyl ether | BFG | 170.2 | 61.5 | |
| Dipropylene glycol monobutyl ether | BFDG | 230.6 | 117 | |
| Propylene glycol monomethyl ether acetate | PMA | 146.0 | 48 | |
| Dialkyl Glycol Ether | Diethylene glycol dimethyl ether | DMDG | 162.0 | 56 |
| Triethylene glycol dimethyl ether | DMTG | 216.0 | 113 | |
| Diethylene glycol diethyl ether | DEDG | 188.9 | 70.8 | |
| Diethylene glycol dibutyl ether | DBDG | 254.6 | 122 | |
| Others | 3-Methoxy-3-methyl-1-butanol | MMB | 174.0 | 68 |
| 3-Methoxy-3-methyl-1-butyl acetate | MMB-Ac | 188.0 | 75.5 |
(*Boiling Point and Flash Point varies according to the manufacturers of the chemicals.)
Glycol ether has both polar and nonpolar characteristics in its molecule. Mixing different glycol ethers enables a design that has the most suitable dissolvability of flux residue with an extensive solubility parameter. While glycol ether itself has a flash point and may be classified as hazardous under the Fire Services Act, with a proper chemicals design it can get away as nonhazardous by adding a certain amount of water. Water dissolved glycol ether becomes insoluble in water at a certain point called a cloud point as the temperature rises. Oil/water separating cleaning chemicals take the advantage of this characteristic to recycle water by isolating and removing the cleaning compounds from the rinse water.
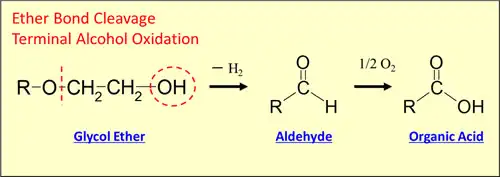
Oxidative decomposition is problem glycol ether may fall into under a heated environment especially when used for deflux chemicals; it accelerates together with organic acids in flux to compose more organic acids including formic acid (Fig 6.2.3), promoting the phenomenon even more. Also since organic acids undermine the material quality with PCB corrosion, electro chemical migration and such, glycol ether needs an appropriate antioxidant to prevent oxidative decomposition in an ordinary usage environment.
Glycol ether based cleaning chemicals are hard to dry out because of their compounds holding a high flash point and boiling point for safety reasons. Thus drying process is common with fast drying rinse chemicals replacing the deflux chemicals. Water or water content alcohol is the most suitable for rinsing glycol ether based deflux chemicals; in order to thoroughly remove ionic substances that PCBs are the least endurable of them remaining on the surface.
Although water is the safest and the most ecological material for rinse, it comes in second in replacing chemicals in narrow gaps, under the electronic components for instance, due to its high surface tension. In addition, much of the residual flux compounds do not dissolve in water that those once solute in deflux chemicals precipitate again, reattaching to the cleaned materials when they contact the rinsing water. These issues can be solved by adding surfactants to deflux chemicals, yet some surfactants may cause rinsing water to bubble, barring its performance. Also, surfactants, easily remained on PCBs, may cause dielectric loss for its hygroscopicity. 5 Besides, water has other issues including microbial contamination and high corrosiveness on metal surfaces e.g. circuits and electrical parts. Designing proper deflux chemicals requires thorough and comprehensive consideration on all such features.
Industrially used water content alcohol consists of mainly ethanol for safety and environmental reasons and a little more than 40 weight percent of water, being nonhazardous under the Fire Services Act. It has such advantages over water as:
a. better rinse property with a less surface tension compared to glycol ether based chemicals,
b. lesser reattachment of precipitated flux residue,
c. consistent circuit characteristics before and after the cleaning process since adding surfactants to deflux chemicals is unnecessary,
d. dry faster than water, equivalent to IPA,
e. mere metal corrosiveness,
f. less risk on microbial contamination because of its bactericidal effect.
Meanwhile, despite of the fact that it is nonhazardous under the Fire Services Act, water content alcohol can only be used in safely designed cleaning equipment because it is flammable similarly to drinkable alcohol such as whisky. 6
Improved, smaller electrical circuits or parts such as multichip modules will demand even better performances of deflux chemicals in order to secure the characteristics and reliability of high-density circuits, by completely removing flux residue and other foreign materials. At the same time, eco-friendliness will have a higher profile in terms of mitigating the energy and environmental burdens.
Establishing the effective deflux system as a whole, by combining chemicals with practical cleaning methods and cleaning equipment, is essential to meet the above-mentioned requirements.
Yuki Akamatsu / Shigeo Hori
1)Tadashi Kubota, Reliable Post-Freon Technology on PCBs (Triceps, 1993)
2)Kogyo Chosakai Publishing, Ready-to-use Cleaning Technology (Kogyo Chosakai Publishing Co., Ltd., 2001)
3)Japan Industrial Conference on Cleaning, Industrial Cleaning Checklist 2009 ed. (Japan Industrial Conference, 2009)
4)Shigeo Hori, Ready-to-Use Cleaning Technology (Kogyo Chosakai Publishing Co., Ltd., 2001) p273.
5)Seihou Terasawa, Reliable Post-Freon Technology on PCBs (Triceps, 1993)
6)Shigeo Hori, Ready-to-use Cleaning Technology (Kogyo Chosakai Publishing Co., Ltd., 2001) p335.
 |
Technial report
| ||
|---|---|---|---|
 |
PCB Mounting Columns
 Technial report | |
| Inquiry |